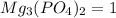Answer:
The stoichiometric coefficient of
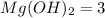
The stoichiometric coefficient of
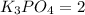
The stoichiometric coefficient of
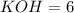
The stoichiometric coefficient of
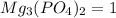
Step-by-step explanation:
The balanced chemical reaction :
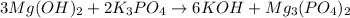
According to reaction, 3 moles of magnesium hydroxide reacts with 2 moles of potassium phosphate to give 6 moles potassium hydroxide and 1 mole of magnesium phosphate as products.
The stoichiometric coefficient of
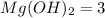
The stoichiometric coefficient of
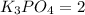
The stoichiometric coefficient of
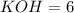
The stoichiometric coefficient of