Answer:
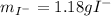
Step-by-step explanation:
Hello,
In this case, the reaction is given as:
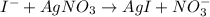
Thus, starting by the yielded grams of silver iodide, we obtain:
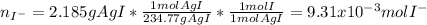
Which correspond to the iodide grams in the silver iodide. In such a way, by means of the law of the conservation of mass, it is known that the grams of each atom MUST remain constant before and after the chemical reaction whereas the moles do not, therefore, the mass of iodine from the silver iodide will equal the mass of iodine present in the soluble iodide, thereby:
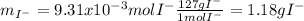
And the rest, correspond to the iodide's metallic cation which is unknown. Such value has sense since it is lower than the initial mass of the soluble iodide which is 1.454g, so 0.272 grams correspond to the unknown cation.
Best regards.