Answer:
The pH of the mixed buffer will be
![([Base])/([Acid])](https://img.qammunity.org/2021/formulas/chemistry/college/a1hmjbd3kibjh2a9xs4w0qrqvijobqltxd.png) = 1.737
= 1.737
Step-by-step explanation:
Buffer is prepared by mixing solutions of KH₂PO₄ and K₂HPO₄
H₂PO₄⁻ ⇄ HPO₄²⁻ + H⁺
Using Henderson equation
⇒ pH = pKa₂ + log
![([Base])/([Acid])](https://img.qammunity.org/2021/formulas/chemistry/college/a1hmjbd3kibjh2a9xs4w0qrqvijobqltxd.png)
⇒ 7.45 = -log(6.2 x 10⁻⁸) + log
![([Base])/([Acid])](https://img.qammunity.org/2021/formulas/chemistry/college/a1hmjbd3kibjh2a9xs4w0qrqvijobqltxd.png)
⇒ log
![([Base])/([Acid])](https://img.qammunity.org/2021/formulas/chemistry/college/a1hmjbd3kibjh2a9xs4w0qrqvijobqltxd.png) = 7.45 - 7.2
= 7.45 - 7.2
⇒
![([Base])/([Acid])](https://img.qammunity.org/2021/formulas/chemistry/college/a1hmjbd3kibjh2a9xs4w0qrqvijobqltxd.png) =
=
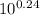
⇒
![([Base])/([Acid])](https://img.qammunity.org/2021/formulas/chemistry/college/a1hmjbd3kibjh2a9xs4w0qrqvijobqltxd.png) = 1.737
= 1.737
So, pH of Buffer solution is equal to 1.737