The volume is reduced to 8.5 L.
Step-by-step explanation:
According to Charles law, the gases has the tendency to expand on heating, and so the volume of the gases increases on increasing the temperature which is measured in Kelvin, and also the volume and temperature are in direct proportion.
Given that:
V1 = Volume 1 = 10 L
T1 = Temperature 1 = 295 K
V2 = ?
T2 = Temperature 2= 250 K
Now Charles law can be mathematically expressed as,
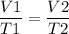
We can rearrange the equation to find V2 as,
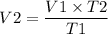
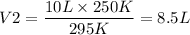
Thus we obtain the volume V2 = 8.5 L which is low when compared to Volume 1 so we can conclude that the volume has reduced