Answer:
Single displacement or reduction/oxidation
Step-by-step explanation:
In a single-displacement reaction, one element exchanges partners with another.
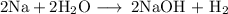
This is a single-displacement reaction, because the element Na exchanges partners with H.
This is also a reduction/oxidation (redox) reaction, because the oxidation number of Na increases from 0 to +1 (oxidation), while the oxidation number of H decreases from +1 to 0 (reduction),
The most common types of reactions are:
Combination
Decomposition
Single displacement
Double displacement
Reduction/oxidation