Answer: 1.
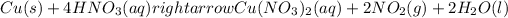 : oxidation reduction
: oxidation reduction
2.
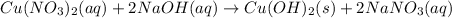 : precipitation
: precipitation
3.
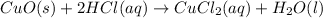 : Double displacement
: Double displacement
Step-by-step explanation:
Oxidation-reduction reaction or redox reaction is defined as the reaction in which oxidation and reduction reactions occur simultaneously.
Oxidation reaction is defined as the reaction in which a substance looses its electrons. The oxidation state of the substance increases.Reduction reaction is defined as the reaction in which a substance gains electrons. The oxidation state of the substance gets reduced.
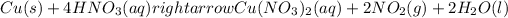
Double displacement reaction is defined as the reaction where exchange of ions takes place. Double displacement reaction in which one of the product remain in solid form are represented by (s) after their chemical formulas. Such double displacement reaction are called as precipitation reaction.
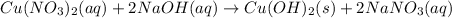
Double displacement reaction is defined as the reaction where exchange of ions takes place.
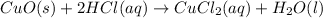
Single displacement reaction is defined as the reaction where more reactive element displaces a less reactive element from its chemical reaction.
Decomposition reaction is defined as the reaction where a single substance breaks down into two or more simpler substances.
Synthesis/Combination reaction is defined as the reaction where substances combine in their elemental state to form a single compound.