Answer:
The chemical formula of the gas is
 .
.
Step-by-step explanation:
The difference between the container with gas and empty container is equal to the mass of the gas.
Let the gas be
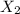
Mass of the gas = m
Molar mass of diatomic gas = M
Pressure of the gas = P = 1.80 atm
Volume of the gas = V = 4.6 L
Temperature of the gas= T = 27.0 C = 27.0 + 273 K = 300 K
Moles of diatomic gas = n =
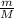
Using ideal gas equation:

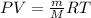
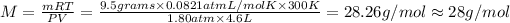
Atomic mass of the element X =
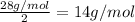
The element is nitrogen and the diatomic gas is
 .
.