Answer:

Step-by-step explanation:
Hello,
In this case, the first step is to compute the oxalic acid dihydrate's moles as shown below, considering its molar mass as 126 g/mol:
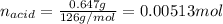
Now, since the undergoing chemical reaction is:
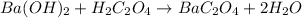
Their molar relationship is 1 to 1, therefore:

Thus, the barium hydroxide's molarity turns out:
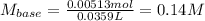
Best regards.