Answer:
The
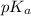 of the X-281 medicne is 4.29.
of the X-281 medicne is 4.29.
Step-by-step explanation:
Concentration of medicine in solution =
![[HA]=c=0.079 M](https://img.qammunity.org/2021/formulas/chemistry/college/f6ek5p1e62rg057xaqpwh9fozgkifyhdmg.png)
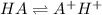
Initially
c 0 0
At equilibrium
(c-x) x x
The expression of an dissociation constant
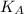 is given by :
is given by :
![K_a=([A^-][H^+])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/high-school/n5xvssrqsuz68kas50gyuv3j3mnhrgjfp7.png)
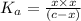 ..[1]
..[1]
The pH of the solution = 2.70
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
![2.70=-\log[H^+]=-\log[x]](https://img.qammunity.org/2021/formulas/chemistry/college/o5s2r0s2jo44wrhbc0io8o2ziwadmr8pu8.png)
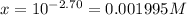 ..[2]
..[2]
Using [2] in [1]
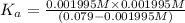
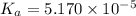
The
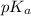 of the medicine :
of the medicine :
![pK_a=-\log[K_a]=-\log[5.170* 10^(-5)]=4.29](https://img.qammunity.org/2021/formulas/chemistry/college/rf8mne6qj2e3naemsjg4w7iz19lbc4eymt.png)
The
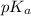 of the X-281 medicne is 4.29.
of the X-281 medicne is 4.29.