Answer: The equilibrium constant for the given reaction is 4.224
Step-by-step explanation:
We are given:
Equilibrium concentration of water = 0.250 M
Equilibrium concentration of hydrogen gas = 0.330 M
Equilibrium concentration of oxygen gas = 0.800 M
For the given chemical reaction:
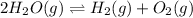
The expression of
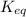 for above reaction follows:
for above reaction follows:
![K_(eq)=([H_2][O_2])/([H_2O]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/477eidk2et7hmtku573dn0ofi7bmc276bm.png)
Putting values in above expression, we get:
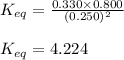
Hence, the equilibrium constant for the given reaction is 4.224