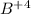Answer : The element is boron and ion is
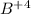
Explanation :
Using Rydberg's Equation:
Where,
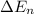 = change in energy
= change in energy
Z = atomic number
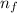 = Higher energy level =
= Higher energy level =
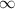
 = Lower energy level = 1
= Lower energy level = 1
Putting the values, in above equation, we get
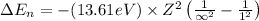
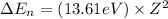 ............(1)
............(1)
As we know that:
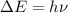
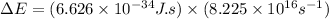
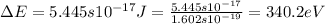 .......(2)
.......(2)
Now equation 1 and 2, we get:
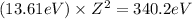
Z = 4.99 ≈ 5
The element is boron that has atomic number 5.
As it has only one electron the charge on the B is (+4) that is,
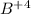
Thus, the element is boron and ion is