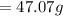Answer:
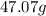
Step-by-step explanation:
500 watts is equivalent to 500J/s
20 minutes is equivalent to 20*60seconds which is 1200 s
330g is equivalent to 330/1000kg which is 0.33kg
The temperature will be (100-23) which is 77°C
The energy will be:
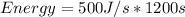
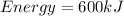
Now we can apply:
Q=
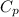 *m*ΔT
*m*ΔT
Q=4.186kJ/kgC * 0.33kg * 77°C
Q=106.37kJ
Energy to boil water is 2260J/kg
2260J/kg*0.33=
so The energy available will be:
=745.8kJ-106.37kJ
=639.43kJ
Now the remaining water will be:
=(1-639.43kJ/745.8kJ)*0.33