Answer:
- Trial and error or algebraic method.
Step-by-step explanation:
There are some very complex reactions which must be balanced using redox methods which would make this a too broad and difficult topic; thus, I must assume that you are talking about simpler equations like synthesis, single replacement, double replacement, decomposition or combustion, which common chemical reations.
The simplest, most of the times is trial and error but, when you have problems with that, you can use an algebraic approach.
Let's do an example. Assume this equation:

Trial and error
1. Balance C:
To make 6 C atoms, put a 6 as coefficient of the CO₂ molecule on the right-hand side
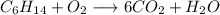
2. Balance H:
To make 14 H atoms, put a 7 as coefficient of the H₂O molecule on the right-hand side:
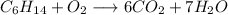
3. Balance O
Now that C and H atoms are balance proceed with O.
There are 6 × 2 + 7 × 1 = 19 atoms of O on the right-hand side. How can you make 19 atoms of O with a O₂ molecule? You can put a fractional coeffcient:
Thus put 19/2 as coeffcient of the O2 molecule on the left side:
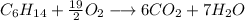
4. Convert to whole numbers
Normally, you would prefer whole numbers as coefficients.
To convert all the coefficients to whole number you just must multiply by the least common denominator of the coefficients, which is 2 because the only fraction is 1/2.
Then, by multiplying the whole equation by 2, you get:
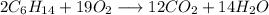
Verify: there are 12 atoms of C on both sides, 28 atoms of H on both sides, and 28 atoms of O on both sides. Hence, the equation is balance.
Algebraic method
Put some generic coefficents, A, B, C, D before each substance:

Write the equations derived from the balance of each kind of atoms:
For C:
For H:
For O:
Assume the value for any of them. The easier is X = 1
Then, from the equations:
- 2Y = 2(6) + 7 = 19 ⇒ Y = 19/2
To have whole coefficients, multiply by the least common denominator: 2:
- X = 2(1) = 2
- Z= 2(6) = 12
- W = 2(7) = 14
- Y = 2(19/2) = 19
Then, replace the letters on the equation with the numbers:
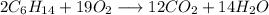
Which is the same equation obtained by trial and error.