Answer:
1-Butene
Step-by-step explanation:
In the final reaction, the
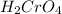 will oxidize the previous molecule to obtain the butanoic acid, therefore the previous molecule has to be primary alcohol: 1-Butanol. The
will oxidize the previous molecule to obtain the butanoic acid, therefore the previous molecule has to be primary alcohol: 1-Butanol. The
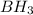 /
/
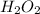 reaction can produce antimarkonikov alcohols therefore the initial molecule has to be an alkene: the 1-Butene.
reaction can produce antimarkonikov alcohols therefore the initial molecule has to be an alkene: the 1-Butene.
To further explanations see the attached image.