The question is incomplete, here is the complete question:
16.1 g of bromine are mixed with 8.42g of chlorine to give an actual yield of 21.1 g of bromine monochloride. Determine the percent yield of the reaction.
Answer: The percent yield of the reaction is 90.71 %.
Step-by-step explanation:
To calculate the number of moles, we use the equation:
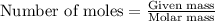 .....(1)
.....(1)
Given mass of bromine gas = 16.1 g
Molar mass of bromine gas = 159.8 g/mol
Putting values in equation 1, we get:
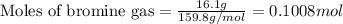
Given mass of chlorine gas = 8.42 g
Molar mass of chlorine gas = 71 g/mol
Putting values in equation 1, we get:
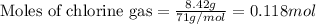
The chemical equation for the reaction of bromine and chlorine gas follows:
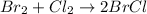
By Stoichiometry of the reaction:
1 mole of bromine gas reacts with 1 mole of chlorine gas
So, 0.1008 moles of bromine gas will react with =
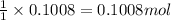 of chlorine gas
of chlorine gas
As, given amount of chlorine gas is more than the required amount. So, it is considered as an excess reagent.
Thus, bromine gas is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
1 mole of bromine gas produces 2 mole of bromine monochloride
So, 0.1008 moles of bromine gas will produce =
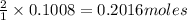 of bromine monochloride
of bromine monochloride
Now, calculating the mass of bromine monochloride from equation 1, we get:
Molar mass of bromine monochloride = 115.36 g/mol
Moles of bromine monochloride = 0.2016 moles
Putting values in equation 1, we get:
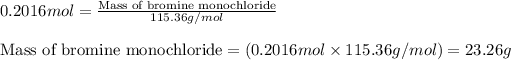
To calculate the percentage yield of bromine monochloride, we use the equation:
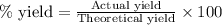
Actual yield of bromine monochloride = 21.1 g
Theoretical yield of bromine monochloride = 23.26 g
Putting values in above equation, we get:
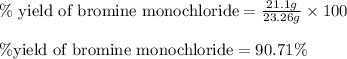
Hence, the percent yield of the reaction is 90.71 %.