Answer:
The mass of the neon gas m = 1.214 kg
Step-by-step explanation:
Pressure = 3 atm = 304 k pa
Volume = 0.57 L = 0.00057

Temperature = 75 °c = 348 K
Universal gas constant = 0.0821
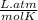
We have to change the unit of this constant. it may be written as
Universal gas constant = 8.314
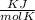
Gas constant for neon =
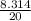 = 0.41
= 0.41
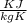
From ideal gas equation,
P V = m R T ------- (1)
We have all the variables except m. so we have to solve this equation for mass (m).
⇒ 304 ×
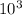 × 0.00057 = m × 0.41 × 348
× 0.00057 = m × 0.41 × 348
⇒ 173.28 = 142.68 × m
⇒ m = 1.214 kg
This is the mass of the neon gas.