Answer: Concentrations of nitrogen gas and hydrogen gas that were reacted initially were 6.5 M and 0.5 respectively
Step-by-step explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
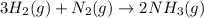
at t= 0 x y 0
tt t= eqm (x-3z) (y-z) 2z
2z = 3.0 M
z= 1.5 M
Initial concentration of
 =
=
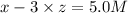
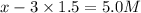
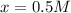
Initial concentration of
 =
=
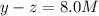
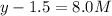
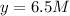
Thus concentrations of nitrogen gas and hydrogen gas that were reacted initially were 6.5 M and 0.5 respectively