Answer:
0.0686 J/g ° C
Step-by-step explanation:
The specific heat capacity is the amount of heat needed to raise 1 gram of a substance by 1° C
We are given temperatures in Kelvin; so we need to convert it to ° C
° C = K - 273.15
For the initial temperature T₁ = 293 K
T₁ = 293 - 273.15 ° C
T₁ = 19.85 ° C
For the final temperature T₂
T₂ = 313 - 273.15 ° C
T₂ = 39.85 ° C
Change in temperature = ΔT = T₂ - T₁
ΔT = 39.85 ° C - 19.85 ° C
ΔT = 20.00 ° C
mass given = 35 g
heat absorbed (q) = 48 J
specific heat (c) = ????
Using the expression:
q = mcΔT
we have:
48 = 35 × c × 20
48 = c × 700
c =
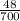
c = 0.0686 J/g ° C