Answer: The molarity of solution is 0.799 M , molality of solution is 1.02 m, mole fraction of camphor is 0.045 and mass percent of camphor in solution is 13.43 %
Step-by-step explanation:
- Calculating the molarity of solution:
To calculate the molarity of solution, we use the equation:
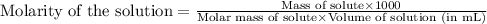
Given mass of camphor = 70.0 g
Molar mass of camphor = 152.2 g/mol
Volume of solution = 575 mL
Putting values in above equation, we get:
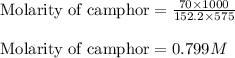
- Calculating the molarity of solution:
To calculate the mass of ethanol, we use the equation:
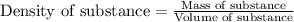
Density of ethanol = 0.785 g/mL
Volume of ethanol = 575 mL
Putting values in above equation, we get:
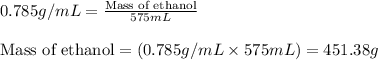
To calculate the molality of solution, we use the equation:
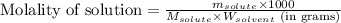
where,
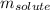 = Given mass of solute (camphor) = 70 g
= Given mass of solute (camphor) = 70 g
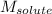 = Molar mass of solute (camphor) = 152.2 g/mol
= Molar mass of solute (camphor) = 152.2 g/mol
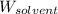 = Mass of solvent (ethanol) = 451.38 g
= Mass of solvent (ethanol) = 451.38 g
Putting values in above equation, we get:
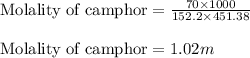
- Calculating the mole fraction of camphor:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
For camphor:
Given mass of camphor = 70 g
Molar mass of camphor = 152.2 g/mol
Putting values in equation 1, we get:
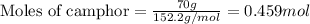
For ethanol:
Given mass of ethanol = 451.38 g
Molar mass of ethanol = 46 g/mol
Putting values in equation 1, we get:
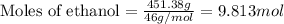
Mole fraction of a substance is given by:
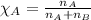
Moles of camphor = 0.459 moles
Total moles = [0.459 + 9.813] = 10.272 moles
Putting values in above equation, we get:
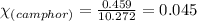 \
\
- Calculating the mass percent of camphor:
To calculate the mass percentage of camphor in solution, we use the equation:
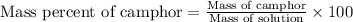
Mass of camphor = 70 g
Mass of solution = [70 + 451.38] = 521.38 g
Putting values in above equation, we get:
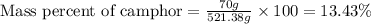
Hence, the molarity of solution is 0.799 M , molality of solution is 1.02 m, mole fraction of camphor is 0.045 and mass percent of camphor in solution is 13.43 %