Answer:
a) YES, this is a minimum standard reduction potential and
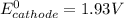
b) NO maximum reduction potential of half cell of cathode.
Step-by-step explanation:
Given that the galvanic cell must provide at least 1.30 V of electrical power . that denotes that
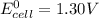
Here, the standard reduction potential of the half-cell reaction of anode is + 0.63 V
i.e
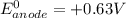
We know that:
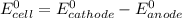
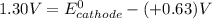
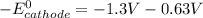
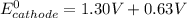
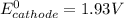
If
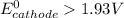 ; then
; then
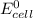 > 1.30 V
> 1.30 V
Also; If
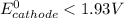 ; then
; then
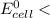 1.30 V
1.30 V
This is because the cell supply minimum electrical power that is 1.30 V , Hence reduction potential of half- cell reaction at cathode will be minimum at 1.93 V
Therefore, from the foregoing, we conclude that: YES, this is a minimum standard reduction potential.
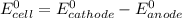
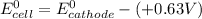
So, if reduction potential value of the half-cell reaction used at cathode is greater than 1.93 (V); we get at least 1.30 V or greater than 1.30 V cell emf.
As such, No maximum reduction potential of half cell of cathode.