Volume of CH3COOH will be needed to neutralize the KOH is 10 ml.
Step-by-step explanation:
In the titration process the concentration of unknown analyte be it acid or base is calculated by the formula:
Macid x Vacid = Mbase x Vbase equation 1
Data given:
volume of base KOH = 20 ml
molarity of base = 2M
Molarity of CH3COOH acid = 4 M
Volume of CH3COOH acid = ?
Putting the values in the equation 1
4 x V acid = 20 x 2
V acid =
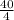
V acid = 10 ml
The neutralization of KOH by CH3COOH will be done by using 10 ml of 4M solution of CH3COOH against 20 ml of 2M KOH.