Answer:
The age of the rock is 800,000 years old
Step-by-step explanation:
The question has a typo, it's impossible to have more radioactive elements in the present compared to the past. The correct question will be "but only contains 250 radioactive parent atoms today"
A radioactive molecule will continuously decay and their mass will keep become lower as time goes. This phenomenon makes radioactive molecule can be used to estimate the age of an object. Half-life is the unit of time needed for radioactive molecules to decay to half of its mass. The formula for the mass remaining will be:
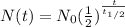
Where
N(t)= number of the molecule remains (250)
N0= number of molecule initially (1000)
t= time elapsed (?)
t1/2= half time (400,000 years)
The calculation will be:
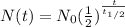
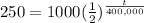
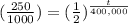
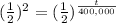
2= t/ 400,000
t= 2*400,000
t= 800,000
The age of the rock is 800,000 years old