Answer:
The data indicates towards a acetophenone molecule (SMILES CC(=O)c1ccccc1). (molecule image is also attached)
Step-by-step explanation:
Let's first calculate the index of hydrogen deficiency (IHD) of the compound, which helps to determine the degree of unsaturation in a molecule. For hydrocarbons, It can be calculated as
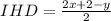
Here, x and y stands for number of carbon and hydrogen atoms respectively.
So,
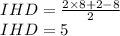
The IR spectrum shows absorbance at 3063 cm⁻¹, this absorbance is associated with the stretching of C-H bond of the alkane group. Strong absorbance band at 1686 cm⁻¹ indicates the presence of conjugated ketone group. The last absorbance mentioned at 1646 cm⁻¹ is usually associated with the stretching of conjugated C=C bond.
The NMR spectrum shows a singlet at 2.6 ppm with the integration of three H atoms. This peak is attributed to the hydrogen of alpha carbon of carbonyl group. Another peak at 7.5 ppm with integration of 5 hydrogen atoms is a characteristic peak of the hydrogen atoms of the phenyl group.
So, all the data indicates towards a acetophenone molecule (SMILES CC(=O)c1ccccc1). IHD value of 5 satisfies a cyclic ring with three double bonds and a C=O bond. Conjugated ketone (1686 cm⁻¹) group is indicated by the IR spectrum and the phenyl group (7.5 ppm) is indicated by the NMR spectrum. The methyl group next to ketone is predicted by both NMR (2.6 ppm) and FTIR (3063, 1646 cm⁻¹) spectrum