Answer:
The final temperature is closer to 0 degrees.
Step-by-step explanation:
Initially we have to use the following formula of heat transfer. Taking into consideration 0,114 cal/g °C as specific heat capacity of the Iron
![0 = (t_(f)-t_(0))*C_(Fe)*M_(Fe) + (t_(f)-t_(0))*C_{H{2O} }*M_{H{2O} \\\\t_(f) = final.temperature\\t_(0) = initial.temperature\\\\C_(Fe) = 0,114 cal/g Celsius\\C_(H2O) = 1 cal/g Celsius\\\\\\M_{H{2O}} = Mass.water\\M_(Fe) = Mass.iron\\]()
By replacing the formula above we have:
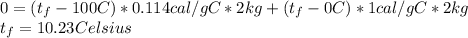
Which makes closer of 0 degrees.