Answer:
The pH is 5.42
Step-by-step explanation:
Given the following;
Ka = 1.3*10^-10 pH =? 0.11m C6H5OH
We would solve the question by using the I.C.E table,
where; I represents Initial Concentration
C represents Change in Concentration
E represents Equilibrium
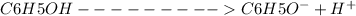
I 0.11m 0 0
C -x +x +x
E 0.11-x x x
The formula, Ka = ([H^{+}] [A^{-}])/[HA]
Ka = ([H+] [C6H5O-])/[C6H5OH]
substituting into the above equation gives;
1.3*10^-10 = [x.x]/[0.11-x]
1.3*10^-10 = x^2/0.11-x
Assume x << 0.11 because C6H5OH dissociation can be neglected. Hence, (0.11-x) approximately equals to 0.11
Therefore,
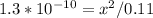
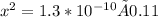
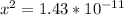
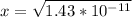
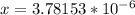
pH = -log(H+)
pH = -log(3.78153*10^-6)
pH = -(-5.42)
pH = 5.42