Answer : The pH of the solution is, 5.24
Explanation :
First we have to calculate the volume of
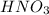
Formula used :

where,
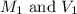 are the initial molarity and volume of
are the initial molarity and volume of
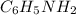 .
.
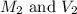 are the final molarity and volume of
are the final molarity and volume of
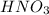 .
.
We are given:
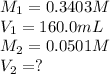
Putting values in above equation, we get:
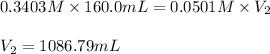
Now we have to calculate the total volume of solution.
Total volume of solution = Volume of
 + Volume of
+ Volume of
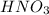
Total volume of solution = 160.0 mL + 1086.79 mL
Total volume of solution = 1246.79 mL
Now we have to calculate the Concentration of salt.
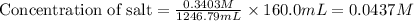
Now we have to calculate the pH of the solution.
At equivalence point,
![pOH=(1)/(2)[pK_w+pK_b+\log C]](https://img.qammunity.org/2021/formulas/chemistry/college/ef0ek8ckx8nzabd8cbe0binpwkjm9ecfn8.png)
![pOH=(1)/(2)[14+4.87+\log (0.0437)]](https://img.qammunity.org/2021/formulas/chemistry/college/44rbe0393wayyidozn0k3l8iznw57i3enk.png)
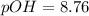
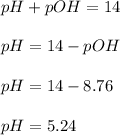
Thus, the pH of the solution is, 5.24