Answer:
6.064 kg
Step-by-step explanation:
The freezing point depression of water is 1.86° C/m
To still freeze at -11.0° C, the molarity of the solution has to be no more than
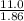 = 5.91 m.
= 5.91 m.
The molecular weight of sucrose is:
12 C = 12 *12 = 144
22 H = 22 * 1 = 22
11 O = 11 * 16 = 176
Total = 342 g/mol
Thus one mole of sucrose has a mass of 342 grams.
However, since there are 3.00 kg of water, you can add
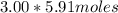 (= 17.73 moles) to the water to get the -11.0° C freezing point depression.
(= 17.73 moles) to the water to get the -11.0° C freezing point depression.
Finally;
mass = number of mole * molar mass
= 17.73 moles * 342 grams / mole = 6063.66 grams
= 6.064 kg