Answer:
126.4 g of
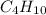 are required
are required
Step-by-step explanation:
Balanced reaction:
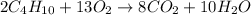
According to balanced reaction-
8 moles of
 are produced from 2 moles of
are produced from 2 moles of
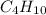
So, 8.70 moles of
 are produced from
are produced from
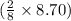 moles of
moles of
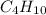 or 2.175 moles of
or 2.175 moles of
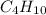
Molar mass of
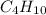 = 58.12 g/mol
= 58.12 g/mol
So, mass of
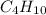 required =
required =
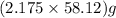 = 126.4 g
= 126.4 g
Hence 126.4 g of
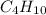 are required
are required