Answer:
0.45 moles of air are in a 3.0 L balloon
Step-by-step explanation:
The given problem can be solved by using unitary method.
Firstly, we have to find out how many breaths are equivalent to 3.0 L. Secondly, we have to find out how many moles of air are supplied corresponding to the calculated number of breaths.
1.2 L = 3 breaths
So, 3.0 L =
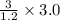 breaths = 7.5 breaths
breaths = 7.5 breaths
1 breath supplies 0.060 moles of air
So, 7.5 breaths supplies
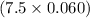 moles of air or 0.45 moles of air.
moles of air or 0.45 moles of air.
Hence, 0.45 moles of air are in a 3.0 L balloon.