Answer:
16.95 grams of this compound must be burn to release 264.90 kJ of heat.
Step-by-step explanation:
The heat of combustion of a compound =
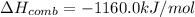
Suppose n moles of compound on combustion gives Q amount of energy
Q = -264.90 kJ
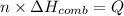
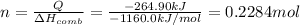
Mass of 0.2284 moles of compound = m
Molar mass of compound = M = 74.23 g/mol
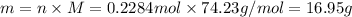
16.95 grams of this compound must be burn to release 264.90 kJ of heat.