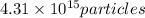Answer:
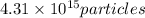
Step-by-step explanation:
Current (I) is the flow of charge (Q) per unit of time (t). The ampere unit is defined as the amount of charge that flows in one second.
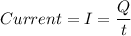
You have the current, 138 µA, and the time, 25.0s, thus you can calculate the charge:
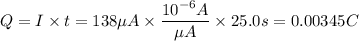
Now calculate the number of particles with a charge of +5eV that have a charge of 0.00345C
The charge of one electron is
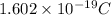
Then, a positve charge equivalent to the magnitude of the charge of 5 electrons (one particle) is:
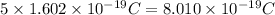
Divide the total charge by the charge of a particle:
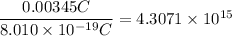
Rounding to 3 significant figures that is