Answer:
A solid precipitate of
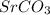 is formed when
is formed when
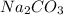 and
and
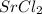 are mixed.
are mixed.
Step-by-step explanation:
A solid precipitate is formed only when an insoluble or sparingly soluble salt is formed through reaction between given reactants
According to solubility rule, all carbonates are insoluble except
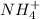 and group IA compounds
and group IA compounds
So, a solid precipitate of
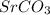 is formed when
is formed when
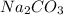 and
and
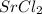 are mixed together
are mixed together
Reactions:
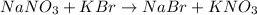 (no precipitation)
(no precipitation)
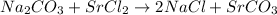 (precipitation)
(precipitation)
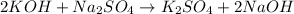 (no precipitation)
(no precipitation)
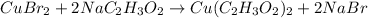 (no precipitation)
(no precipitation)