Answer:
a.) What is the equilibrium concentration of CO at 1000 K? : 0.0130M
b.) What is the equilibrium concentration of Cl₂ at 1000 K?: 0.0410M
c.) What is the equilibrium concentration of COCl₂ at 1000 K?: 0.136M
Step-by-step explanation:
The complete question is:
- Kc= 255 at 1000K for reaction CO(g) + Cl₂(g) ⇄ COCl₂(g). If a mixture initially contains a CO concentration of 0.1490M and a Cl₂ concentration of 0.177M at 1000K, what are the equilibrium concentration of CO, Cl₂ and COCl₂ at 1000K?
a.) What is the equilibrium concentration of CO at 1000 K?
b.) What is the equilibrium concentration of Cl₂ at 1000 K?
c.) What is the equilibrium concentration of COCl₂ at 1000 K?
Solution
1. Write the equilibrium equation
CO(g) + Cl₂(g) ⇄ COCl₂(g)
2. Build the ICE table (initial, change, equilibrium) table
Molar concentrations:
CO(g) + Cl₂(g) ⇄ COCl₂(g)
I 0.1490 0.177 0
C -x -x +x
E 0.01490 - x 0.177 - x x
3. Write the equation of the constant of equilibrium
![K_c=([COCl_2])/([CO][Cl_2)}](https://img.qammunity.org/2021/formulas/chemistry/college/zq3uyirzx929bvswmnhfhuvaw2h24chn8p.png)

4. Solve the equation
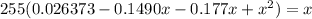
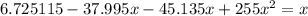
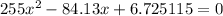
Use the quadratic equation to obtain:
It cannot be greater than 0.1490, thus the correct solution is 0.136
And the concentrations are:
a.) Concentration of CO: 0.1490M - 0.136M = 0.0130M
b.) Concentration of Cl₂: 0.177M - 0.136M = 0.0410M
c.) Concentration of COCl₂: 0.136M