Answer:
The final temperature of the mixture is = 42.14 °C
Step-by-step explanation:
Mass of the aluminium
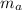 = 101 gm
= 101 gm
Specific heat for aluminium
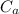 = 0.9
= 0.9
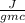
Temperature of aluminium
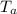 = 100.1 °C
= 100.1 °C
Mass of water
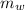 = 48.9 gm
= 48.9 gm
Specific heat for water
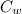 = 4.2
= 4.2
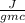
Initial temperature of water
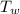 = 16.5 °C
= 16.5 °C
From energy balance principal when the aluminium piece is heated and dropped in to the water , the final temperature of aluminium & the water becomes equal.
From the energy conservation principal
Heat lost from the aluminium piece = heat gain by the water
⇒
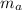
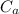 (
(
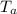 -
-
 ) =
) =
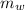
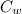 (
(
 -
-
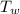 ) ----------- (1)
) ----------- (1)
Put all the values in equation (1)
⇒ 101 × 0.9 × (100.1 -
 ) = 48.9 × 4.2 × (
) = 48.9 × 4.2 × (
 - 16.5 )
- 16.5 )
⇒ 90.9 × (100.1 -
 ) = 205.38 × (
) = 205.38 × (
 - 16.5 )
- 16.5 )
⇒ 100.1 -
 = 2.26 (
= 2.26 (
 - 16.5 )
- 16.5 )
⇒ 100.1 -
 = 2.26
= 2.26
 - 37.28
- 37.28
⇒ 3.26
 = 137.38
= 137.38
⇒
 = 42.14 °C
= 42.14 °C
Therefore the final temperature of the mixture is = 42.14 °C