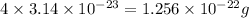Mass of 4 molecules of fluorine atom is found to be =
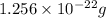
Step-by-step explanation:
In order to determine the mass of one molecule of fluorine atom,We must divide the molar mass of fluorine by the Avogadro's number.
This will get the mass of one molecule.
Molar mass of Fluorine atom is 18.9 g/mol
Mass of one fluorine atom =
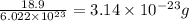
Multiplying the answer by 4, we will get the mass of 4 molecules of fluorine atom.
Mass of 4 molecules of fluorine atom =