10.53 grams is the theoretical yield of CaF2 for this experiment.
Step-by-step explanation:
From the reaction it can be found that Ca reacts with excess of fluoride gas to form calcium fluoride. Thus, it can be concluded that calcium is limiting reagent as product formation depends on the concentration of calcium in the reaction as reactant.
The balanced chemical reaction is :
Ca + F2 ⇒ CaF2
Thus one mole of calcium reacted to form one mole of calcium fluoride.
Number of moles of calcium fluoride can be obtained by using the formula;
number of moles=
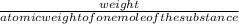
=
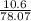
= 0.135 moles of CaF2 is formed.
So,
1 mole of calcium reacts to give 1 mole of CaF2
x moles of calcium will give 0.135 moles of CaF2
Hence, 0.135 moles of CaF2 is formed
the weight formed will be given as 0.135 x 78.07
= 10.53 grams
The theoretical yield is the amount of product formed by complete conversion of limiting reactant. Thus, 10.53 grams is the theoretical yield of CaF2 in the reaction.