Answer:
a. The maximum volume of 0.143 M HCl required is 154.4 mL.
b. The maximum volume of 0.143 M HCl required is 135.7 mL.
Step-by-step explanation:
a.
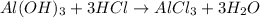
Mass of aluminum hydroxide = 350 mg = 0.350 g ( 1mg = 0.001 g)
Moles of aluminum hydroxide =
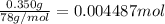
According to reaction ,3 moles of HCl neutralize 1 mole of aluminum hydroxide.Then 0.004487 mole of aluminum hydroxide will be neutralize by :
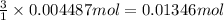 of HCl.
of HCl.
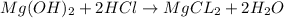
Mass of magnesium hydroxide = 250 mg = 0.250 g ( 1mg = 0.001 g)
Moles of magnesium hydroxide =
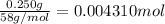
According to reaction ,2 moles of HCl neutralize 1 mole of magnesium hydroxide.Then 0.004310 mole of magnesium hydroxide will be neutralize by :
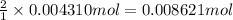 of HCl.
of HCl.
Total moles of HCl required to neutralize both :
0.01346 mol + 0.008621 mol = 0.02208 mol
Molarity of the HCL solution = 0.143 M
Volume of the solution = V
![Molarity=\frac{\text{Total moles of HCl}{\text{Volume in Liter}}]()
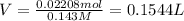
1 L = 1000 mL
0.1544 L = 154.4 mL
The maximum volume of 0.143 M HCl required is 154.4 mL.
b.
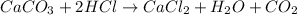
Mass of calcium carbonate = 970mg = 0.970 g ( 1mg = 0.001 g)
Moles of calcium carbonate =
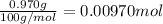
According to reaction ,2 moles of HCl neutralize 1 mole of calcium carbonate.Then 0.00970 mole of calcium carbonate will be neutralize by :
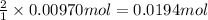 of HCl.
of HCl.
Total moles of HCl required to neutralize calcium carbonate : 0.0194 mol
Molarity of the HCL solution = 0.143 M
Volume of the solution = V
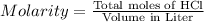
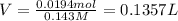
1 L = 1000 mL
0.1357 L = 135.7 mL
The maximum volume of 0.143 M HCl required is 135.7 mL.