Answer : The specific heat (J/g-K) of this substance is, 0.780 J/g.K
Explanation :
Molar heat capacity : It is defined as the amount of heat absorbed by one mole of a substance to raise its temperature by one degree Celsius.
1 mole of substance releases heat = 92.1 J/K
As we are given, molar mass of unknown substance is, 118 g/mol that means, the mass of 1 mole of substance is, 118 g.
As, 118 g of substance releases heat = 92.1 J/K
So, 1 g of substance releases heat =
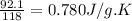
Thus, the specific heat (J/g-K) of this substance is, 0.780 J/g.K