Step-by-step explanation:
First, we will convert 200 ml into kg as follows.
200 ml = 0.2 kg
Now, we will take the density of water as 1 g/ml.
And, dT =
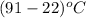
=
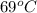
Also, we know that specific heat of water is 4.186
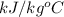 . Therefore, we will calculate the heat energy as follows.
. Therefore, we will calculate the heat energy as follows.
q =
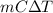
=
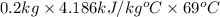
= 5.77 kJ
Now, we will calculate the power delivered as follows.
P =
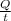
=
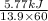
= 69.1 W
It is given that efficiency is 75%. Therefore, input power will be calculated as follows.
![P_(input) = (69.1)/(75) * 100]()
= 92.13 W
Now, we will calculate the current as follows.
P =

I =
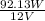
= 7.67 A
Therefore, we can conclude that current drawn from the car's 12-V battery is 7.67 A.