Answer: Final temperature of a gold nugget will be 297 K
Step-by-step explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
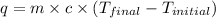
q = heat released = -4.85 kJ = -4850 J
m = mass of metal = 376 g
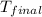 = final temperature of metal = ?
= final temperature of metal = ?
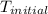 = initial temperature of metal = 398 K
= initial temperature of metal = 398 K
c = specific heat of metal =
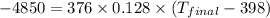
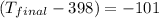
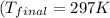
Thus the final temperature of a gold nugget will be 297 K