Answer: The concentration of X is
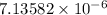 and the concentration of Y is
and the concentration of Y is
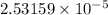 .
.
Explanation:
Since we have given that
At 272 nm, absorbance = 0.215
At 327 nm, absorbance = 0.191
As we have given that
Compound X Compound Y
272 16400 3870
327 3990 6420
So, our equations becomes
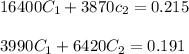
By solving these two equations, we get that

Hence, the concentration of X is
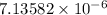 and the concentration of Y is
and the concentration of Y is
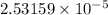 .
.