Answer:
0.0156 atm
Step-by-step explanation:
Let the partial pressure of water vapor be
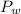 .
.
Given:
Total pressure of air sample is,
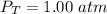
Partial pressure of nitrogen is,
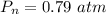
Partial pressure of oxygen is,
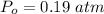
Partial pressure of all other gases is,
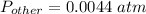
From Dalton's law of partial pressure, we know that, the total pressure of a mixture of gases is equal to sum of partial pressure of each individual gas.
So, total pressure of air sample is equal to the sum of partial pressure of nitrogen, oxygen, other gases and water vapor.
Therefore, framing in equation form, we have:
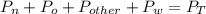
Plug in the given values and solve for
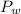 . This gives,
. This gives,
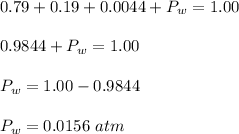
Therefore, the partial pressure of water vapor in an air sample is 0.0156 atm.