Answer:
The velocity of electrons is 1617.27 m/s.
Step-by-step explanation:
Given that,
Wavelength of light,
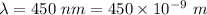
Mass of an electron,
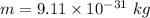
We need to find the velocity of electrons. It can be given by using De-Broglie wavelength such that :
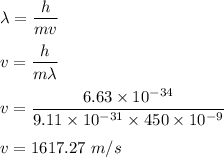
So, the velocity of electrons is 1617.27 m/s. Hence, this is the required solution.