Answer: The pH of the mixture at 0°C is 4.68
Step-by-step explanation:
To calculate the pH at 0°C, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
![pH=pK_a+\log(([CH_3COONa])/([CH_3COOH]))](https://img.qammunity.org/2021/formulas/chemistry/college/i4ov34fhvz4xankmdxm0vt8u79xc3cnnvx.png)
We are given:
 = negative logarithm of acid dissociation constant of acetic acid at 0°C = 4.78
= negative logarithm of acid dissociation constant of acetic acid at 0°C = 4.78
![[CH_3COONa]=0.190M](https://img.qammunity.org/2021/formulas/chemistry/college/5vapj7ufgjv9yglsm63i4hqbycdktww1td.png)
![[CH_3COOH]=0.239M](https://img.qammunity.org/2021/formulas/chemistry/college/jjhdsvxbejor5pu5ds67bv11yi5i64skx8.png)
pH = ?
Putting values in above equation, we get:
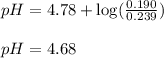
Hence, the pH of the mixture at 0°C is 4.68