The question is incomplete, complete question is :
Calculate the change in enthalpy associated with the combustion of 492.2 g of sulfur. Report your answer with four significant figures. Do not include a comma in your answer.
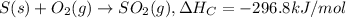
Answer:
-4,565 kJ the change in enthalpy associated with the combustion of 492.2 grams of sulfur.
Step-by-step explanation:
Enthalpy change when 1 mole of sulfur is combusted = -296.8 kJ
Mass of sulfur = 492.2 g
Moles of sulfur =
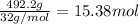
Enthalpy change when 15.38 moles of sulfur:
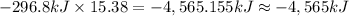
-4,565 kJ the change in enthalpy associated with the combustion of 492.2 grams of sulfur.