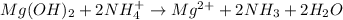Answer:
Net ionic:
Step-by-step explanation:
 in
in
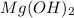 reacts with
reacts with
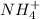 to form
to form
 and
and
 .
.
Due to this acid-base reaction,
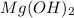 become soluble whenever
become soluble whenever
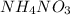 is added to suspension of
is added to suspension of
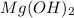 .
.
In this reaction,
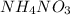 acts as an acid and
acts as an acid and
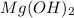 acts as a base.
acts as a base.
Molecular equation:
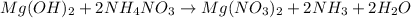
Total ionic:
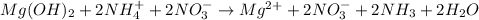
Net ionic: