Step-by-step explanation:
It is known that more is the number of bonds present within two carbon atoms then more strongly they are held together. Therefore, more is the overlapping taking place and smaller will be the length of carbon-carbon bond.
Hence, single bonds are larger in length, double bond is smaller in length as compared to a single bond and a triple bond is the smallest in length.
For example, in ethane (
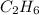 ) there are all single bonds.
) there are all single bonds.
In ethene (
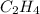 ), there is one double bond present between the carbon atoms.
), there is one double bond present between the carbon atoms.
In ethyne (
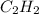 ), there is a triple bond present between the carbon atoms.
), there is a triple bond present between the carbon atoms.
Thus, we can conclude that given compounds are ranked in increasing order by the length of the carbon–carbon bond as follows.
ethyne (
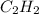 ) < ethene (
) < ethene (
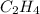 ) < ethane (
) < ethane (
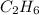 )
)