Answer : The molarity of the chloride ion in the water is, 5.75 M
Explanation :
As we are given that 16.6 % chloride ion that means 16.6 grams of chloride ion present 100 grams of solution.
First we have to calculate the volume of solution.
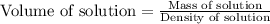
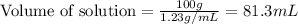
Now we have to calculate the molarity of chloride ion.
Molarity : It is defined as the number of moles of solute present in one liter of volume of solution.
Formula used :
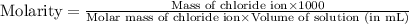
Now put all the given values in this formula, we get:
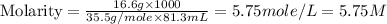
Thus, the molarity of the chloride ion in the water is, 5.75 M