Answer:
The new temperature of the water bath 32.0°C.
Step-by-step explanation:
Mass of water in water bath ,m= 8.10 kg = 8100 g ( 1kg = 1000g)
Initial temperature of the water =
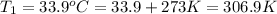
Final temperature of the water =

Specific heat capacity of water under these conditions = c = 4.18 J/gK
Amount of energy lost by water = -Q = -69.0 kJ = -69.0 × 1000 J
( 1kJ=1000 J)
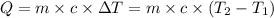
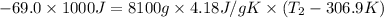
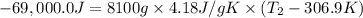
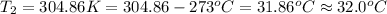
The new temperature of the water bath 32.0°C.