Answer: The specific heat of calorimeter is 30.68 J/g°C
Step-by-step explanation:
When hot water is added to the calorimeter, the amount of heat released by the hot water will be equal to the amount of heat absorbed by cold water and calorimeter.
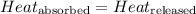
The equation used to calculate heat released or absorbed follows:
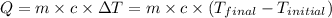
-T_2)](https://img.qammunity.org/2021/formulas/chemistry/college/b3hgz2a9ja4t1f8xbgpvmllco783k2digv.png) ......(1)
......(1)
where,
q = heat absorbed or released
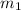 = mass of hot water = 46.7 g
= mass of hot water = 46.7 g
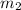 = mass of cold water = 45.33 g
= mass of cold water = 45.33 g
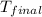 = final temperature = 59.4°C
= final temperature = 59.4°C
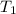 = initial temperature of hot water = 80.6°C
= initial temperature of hot water = 80.6°C
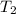 = initial temperature of cold water = 40.6°C
= initial temperature of cold water = 40.6°C
 = specific heat of hot water = 4.184 J/g°C
= specific heat of hot water = 4.184 J/g°C
 = specific heat of cold water = 4.184 J/g°C
= specific heat of cold water = 4.184 J/g°C
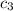 = specific heat of calorimeter = ? J/g°C
= specific heat of calorimeter = ? J/g°C
Putting values in equation 1, we get:
](https://img.qammunity.org/2021/formulas/chemistry/college/r9prgecped880tz4cjsq7u766fuk37ffkk.png)
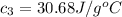
Hence, the specific heat of calorimeter is 30.68 J/g°C